
Iron shares many properties of other transition metals, including the other group 8 elements, ruthenium and osmium. Ĭhemically, the most common oxidation states of iron are iron(II) and iron(III). Iron is also the metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals. To maintain the necessary levels, human iron metabolism requires a minimum of iron in the diet. These two proteins play essential roles in vertebrate metabolism, respectively oxygen transport by blood and oxygen storage in muscles. The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in hemoglobin and myoglobin. Although iron readily reacts, high purity iron, called electrolytic iron, has better corrosion resistance. Unlike the oxides of some other metals, that form passivating layers, rust occupies more volume than the metal and thus flakes off, exposing fresh surfaces for corrosion. However, iron reacts readily with oxygen and water to give brown to black hydrated iron oxides, commonly known as rust. Pristine and smooth pure iron surfaces are mirror-like silvery-gray. The iron and steel industry is thus very important economically, and iron is the cheapest metal, with a price of a few dollars per kilogram or per pound (see Metal#uses). In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, because of their mechanical properties and low cost. That event is considered the transition from the Bronze Age to the Iron Age. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching 1,500 ☌ (2,730 ☏) or higher, about 500 ☌ (932 ☏) higher than that required to smelt copper. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. It is the fourth most common element in the Earth's crust. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core.
#IRON ATOMIC NUMBER SERIES#
It is a metal that belongs to the first transition series and group 8 of the periodic table. Phenom., 1980, 21, 275.Iron ( / ˈ aɪ ər n/) is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26.
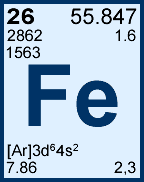
Mårtensson, "Core-Level Binding Energies in Metals," J. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5). The data are adapted from references 1-3. I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules relative to the Fermi level for metals and relative to the top of the valence band for semiconductors.


All values of electron binding energies are given in eV. 1967, 47, 1300.Įlectron binding energies Electron binding energies for iron. These effective nuclear charges, Z eff, are adapted from the following references:


 0 kommentar(er)
0 kommentar(er)
